Posters
2024
2023
2019
To be added soon
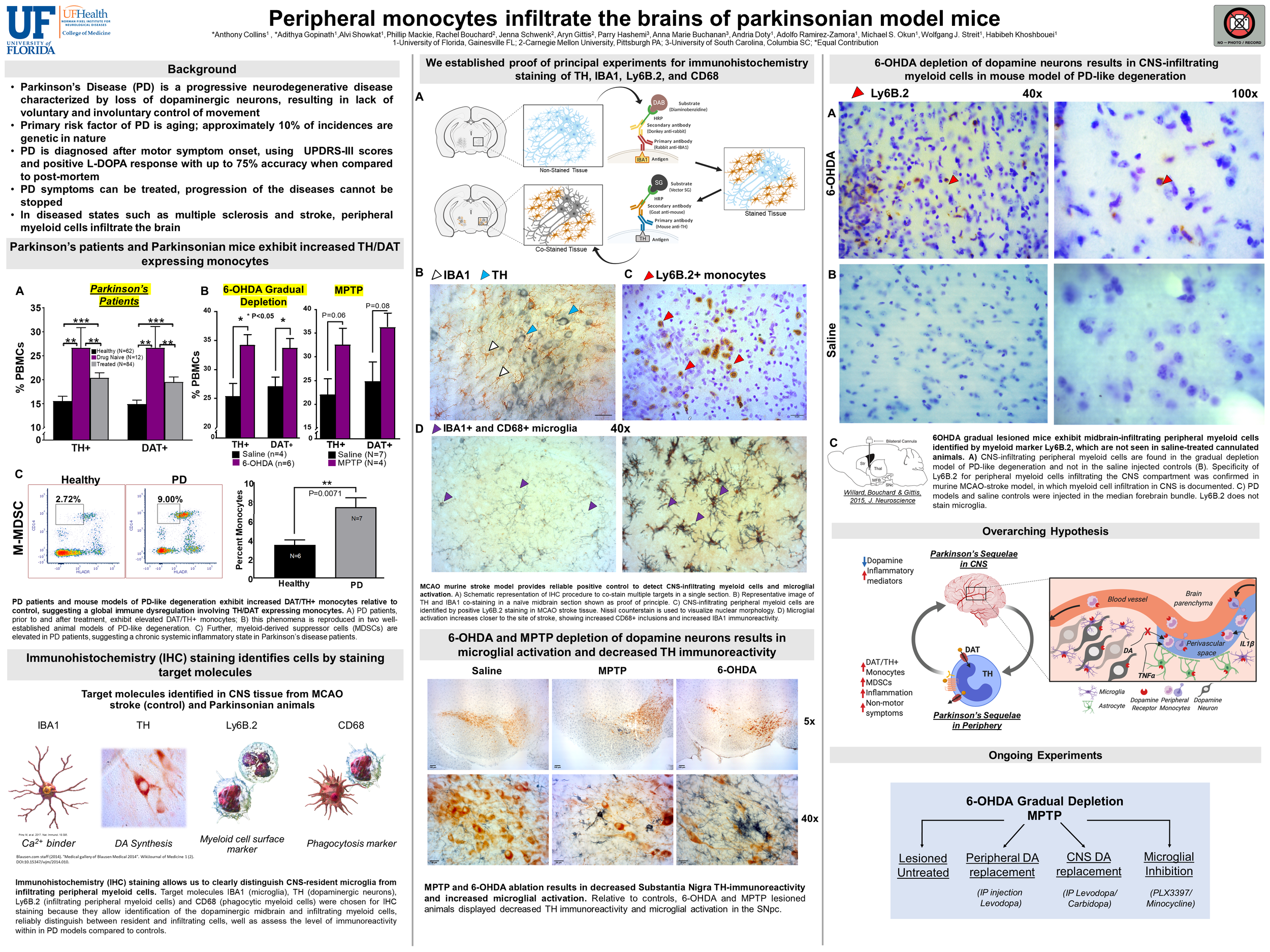
Introduction: Parkinson’s disease (PD) is characterized by loss of substantia nigra (SN) dopaminergic neurons, resulting in a reduction of CNS dopamine transmission and impairment of motor control. PD is believed to be a systemic disease affecting both central and peripheral dopamine transmission, however, its effects in the periphery are still being characterized. Peripheral immune cells express dopaminergic proteins such as dopamine transporter (DAT) and tyrosine hydroxylase (TH) that allow them to regulate and synthesize dopamine. Using a novel flow cytometry panel, we found that in PD patients and in a mouse model of PD, there is an increase in DAT/TH positive monocytes in peripheral blood. In various disease states, such as multiple sclerosis and stroke, microglial activation occurs and peripheral monocytes infiltrate the brain, but it is unknown whether monocytes infiltrate the brains of parkinsonian mice. Objective: We hypothesized that peripheral monocytes cells infiltrate the midbrains of parkinsonian mouse models. Methods: We used immunohistochemistry to conduct a series of proof of principle experiments using middle cerebral artery occlusion (MCAO) as a positive control for monocyte infiltration and microglial activation. We then replicated these methods in two animal models of PD: gradual dopamine depletion by 6-OHDA and acute MPTP lesioning of midbrain dopamine neurons. Results: Similar to the positive control group (MCAO sections), in both animal models of PD, the number of activated microglia within the area of pathology was increased. Both Parkinsonian lesions showed decreased TH immunoreactivity compared to saline injected controls. Of interest, 6-OHDA gradual lesions showed Ly6B.2 positive staining in the midbrain, while MPTP-lesioned brains did not. Nissl staining of Ly6B.2 positive cells shows that both peripheral monocytes and neutrophils, based on nuclear morphology, infiltrate the brain in stroke and 6-OHDA gradually depleted mice. Conclusions: Gradual depletion via 6-OHDA confirms our hypothesis that monocytes infiltrate the CNS, while MPTP lesioning does not. Experiments are ongoing to explore what other immune cells might infiltrate the midbrain under conditions of CNS dopamine depletion, and whether the degree of dopamine depletion affects intensity of immune cell infiltration in Parkinson’s disease.
The dopamine transporter (DAT) regulates dopamine neurotransmission via reuptake of dopamine released into the extracellular space. Interactions with partner proteins alter DAT function and thereby dynamically shape dopaminergic tone important for normal brain function. However, the extent and nature of these interactions are incompletely understood. Here, we describe a novel physical and functional interaction between DAT and the voltage-gated K+ channel Kv2.1 (potassium voltage-gated channel subfamily B member 1 or KCNB1). To examine the functional consequences of this interaction, we employed a combination of immunohistochemistry, immunofluorescence live-cell microscopy, co-immunoprecipitation, and electrophysiological approaches. Consistent with previous reports, we found Kv2.1 is trafficked to membrane-bound clusters observed both in vivo and in vitro in rodent dopamine neurons. Our data provide evidence that clustered Kv2.1 channels decrease DAT’s lateral mobility and inhibit its internalization, while also decreasing canonical transporter activity by altering DAT’s conformational equilibrium. These results suggest that Kv2.1 clusters exert a spatially discrete homeostatic braking mechanism on DAT by inducing a relative increase in inward-facing transporters. Given recent reports of Kv2.1 dysregulation in neurological disorders, it is possible that alterations in the functional interaction between DAT and Kv2.1 affect dopamine neuron activity.
Dopamine neurotransmission plays a significant role in a plethora of behaviors from learning and memory, to motivation and addiction. Dopaminergic information is transmitted by consensus through synchrony (Beeler and Dreyer, 2019). The spatial temporal kinetics of dopamine signaling, comprising diffusion and synaptic transmission, are heavily regulated by dopamine transporter. Therefore, we hypothesized that perturbations in dopamine transporter activity will induce aberrant synchrony between dopaminergic neurons that alters the structure of local dopaminergic and GABAergic networks. To examine dopamine transporter regulation of GABAergic networks in the ventral midbrain regions, neurons were transduced with an AAV5 containing the genetically encoded calcium indicator GCaMP6f. Neuronal somas were segmented, and time resolved calcium signals were used as a proxy for neuronal activity. Networks were constructed by the top 15% strongest temporal functional connections at baseline (aCSF) before and after dopamine transporter activation through Spearman’s rank correlation coefficient. In GABA networks, methamphetamine (a dopamine transporter activator) exposure produced a D2 receptor-sensitive network suppression and induction of subsets of strongly connected neurons to drop out from the network. Furthermore, methamphetamine altered the time course of network assortativity of GABA networks (n = 71 to 134 neurons, 6 independent experiments, one-way ANOVA with Tukey’s post-hoc multiple comparisons, p < 0.05). To examine dopamine transporter regulation of dopaminergic networks, 200 μm coronal slices were prepared from the ventral midbrain comprising both the dopaminergic neuron rich regions substantia nigra pars compacta (SNc) and ventral tegmental area (VTA) of male and female mice. In both regions and sexes, dopamine transporter activation by methamphetamine increased the variance of functional connectivity that persisted even after blockade of GABA, glutamate and dopamine receptors signaling but sensitive to dopamine transporter blockade (n = 5 to 11 slices from 1-3 animals per group, one-way ANOVA with Tukey’s post-hoc multiple comparisons, p < 0.05). Since this preparation is devoid of input from other brain regions, the induction of functional connectivity spikes is likely due to the intrinsic properties of dopaminergic neurons to self-organize their activity. Therefore, dopamine transporter is a fundamental regulator of dopaminergic synchrony, exhibiting cascading effects across both local dopaminergic and GABAergic networks. Our data reveal the central role of dopamine transporter activity in functional network modulation.
Introduction: Parkinson’s disease (PD) is characterized by loss of substantia nigra (SN) dopaminergic neurons, resulting in reduced CNS dopamine transmission. PD is widely thought to start in the periphery; however, whether and how PD affects peripheral dopamine transmission remains unknown. Peripheral immune cells express key dopaminergic proteins, including dopamine transporter (DAT) and tyrosine hydroxylase (TH). Therefore, it is possible that the peripheral dopamine system is affected by PD pathology. Objective: We hypothesized that peripheral immune cells of PD patients exhibit dysregulated dopamine homeostasis. Methods/Results: Using flow cytometry we found human peripheral blood mononuclear cells (PBMCs) constitutively express DAT and TH with 90% of CD14+ cells expressing both markers. Upon examining PBMCs of PD patients receiving a variety of treatments, we found that DAT/TH positive PBMCs are elevated in PD patients compared to healthy controls (n=67 independent biological replicates, P<0.05) irrespective of the treatment modality applied. Importantly, in drug naïve patients diagnosed with PD we observed the highest increase in DAT/TH positive monocytes (n=5, P<0.05) compared to both treated PD patients and healthy individuals, suggesting observed changes correlate with disease pathology and not treatment interventions. Collectively, these data suggest a system-wide dysregulation of the dopamine system on peripheral immune cells in PD. To further understand the functional consequences of increased DAT+/TH+ PBMCs, we asked if DAT function was altered on PD monocyte-derived cells. Live cell fluorescence microscopy and biochemical analysis confirmed healthy human monocyte-derived macrophages (MDM) express membrane-localized, canonically functional DAT (Km=3.2mM). Surprisingly, relative to healthy age-matched controls, PD patients’ MDMs exhibited dramatically elevated DAT-mediated substrate uptake and increased membrane localization (p<0.0001, n=5 biological replicates). Conclusions: Taken together, these data are consistent with the interpretation that in PD peripheral dopamine homeostasis is dysregulated. Importantly, this presents PBMC DAT/TH as a potential biomarker for PD and suggests the peripheral dopamine system is functionally linked to the CNS dopamine system. Future work will aim to validate this biomarker and investigate the mechanistic connection between peripheral-CNS dopamine systems to elucidate the pathophysiological role of dysregulated monocyte dopamine transmission in PD.
During aging humans lose brain dopamine neurons, but there is a regional variability and not all dopamine neurons exhibit vulnerability to neurodegeneration. Microglia are responsible for maintaining tissue homeostasis, neuronal support and protection. However, with aging, microglial cells are known to become senescent and likely lose some of their functional abilities. Since aging is the major risk factor for neurodegenerative diseases, including Parkinson’s disease, we hypothesized that the ratio of microglia to dopaminergic neurons as well as microglial heterogeneity change with aging in the more vulnerable substantia nigra pars compacta (SNc) but not in the ventral tegmental area (VTA). To address this hypothesis, we conducted stereological analyses to measure age-dependent changes in the number of microglia and dopaminergic neurons in the SNc and VTA of 1-, 6-, 9-, 18- and 24- month- old C57BL/J6 male mice. For quantification of the anatomical features of microglia, coronal sections of the midbrain were stained with tyrosine hydroxylase (TH) and Iba1 and performed stereological image analysis. Contrary to our hypothesis, in both brain regions, microglia increased in aged mice, whereas the number of TH+ cells decrease after 1 month. Quantitative morphometric analyses revealed microglial complexity and projection area declined with aging while cell body size increased. Surprisingly, the contact sites between microglia and dopaminergic neurons in both regions increased in aged mice, suggesting an aging-dependent increase in microglial physical support of dopamine neurons. To assess neurotrophic expression of dopaminergic neuron, BDNF and TH mRNA were quantified. Results indicated the ratio of BDNF to TH decreased with aging in the SNc, but not the VTA. Furthermore, to assess gait deficits with aging, ventral plane imaging (DigiGait) was utilized. Gait analysis indicated aging-dependent changes in mice gait indices. In conclusion, increases in microglial cell number, ratio of microglia to dopamine neurons, and physical contact sites suggest these innate biological mechanisms may compensate for the aging-dependent decline of microglia complexity (senescence) for continued neuronal support in aging within the SNc and VTA.
2018
Repeated exposure to low to moderate doses of methamphetamine have been shown to induce behavioral sensitization, including increased locomotor responses to the same dose of drug. While the exact mechanism of methamphetamine-induced behavioral sensitization is less clear, increased sensitivity of dopaminergic signaling including decreased activity of inhibitory D2 autoreceptors (D2R) is one proposed mechanism. We and other have shown acutely administered methamphetamine increases the frequency of firing activity of dopamine neurons and stimulates dopamine efflux via the dopamine transporter. Increased extracellular dopamine in the ventral tegmental area (VTA) in turn activates the inhibitory D2Rs resulting in feedback inhibition and decreased activity of dopamine neurons. In this study, we investigated the effect of repeated methamphetamine exposure (2mg/kg/7days) on the baseline activity of VTA dopamine neurons. Whole-cell slice recordings in VTA dopamine neurons of methamphetamine vs. saline treated mice revealed that sub-chronic, systemic exposure to methamphetamine increased the baseline excitability of VTA dopamine neurons, such that methamphetamine-treated animals showed a more depolarized membrane potential and increased spontaneous firing activity. To our surprise, the “sag” current, as measured by the Ih-mediated current after application of hyperpolarizing current pulses, showed no change of methamphetamine vs. saline treated animals, suggesting no changes in the inhibitory function of the neurons. Analysis of current evoked single action potential (AP) properties revealed that sub-chronic methamphetamine exposure narrowed the AP width, increased the AP amplitude, and decreased the depolarization input resistance. Further analysis revealed that these changes in basal neuronal activity were coupled to increased activity of Ca2+ channel activity as well as the frequency and amplitude of intracellular Ca2+ transients in VTA dopamine neurons. These findings suggest that in the absence of D2R activation by high levels of dopamine, sub-chronic exposure methamphetamine exposure also alters the intrinsic, baseline firing activity of dopamine neurons in a Ca2+ - dependent manner.
Parkinson’s disease (PD) is characterized by loss of substantia nigra (SN) dopaminergic neurons, resulting in reduced CNS dopamine transmission. PD is widely thought to start in the periphery; however, whether and how PD affects peripheral dopamine transmission remains unknown. Peripheral immune cells express key dopaminergic proteins, including dopamine transporter (DAT) and tyrosine hydroxylase (TH). Therefore, it is possible that the peripheral dopamine system is affected by PD pathology. Objective: We hypothesized that peripheral immune cells of PD patients exhibit dysregulated dopamine homeostasis. Methods/Results: Using flow cytometry we found human peripheral blood mononuclear cells (PBMCs) constitutively express DAT and TH with 90% of CD14+ cells expressing both markers. Upon examining PBMCs of PD patients receiving a variety of treatments, we found that DAT/TH positive PBMCs are elevated in PD patients compared to healthy controls (n=67 independent biological replicates, P<0.05) irrespective of the treatment modality applied. Importantly, in drug naïve patients diagnosed with PD we observed the highest increase in DAT/TH positive monocytes (n=5, P<0.05) compared to both treated PD patients and healthy individuals, suggesting observed changes correlate with disease pathology and not treatment interventions. Collectively, these data suggest a system-wide dysregulation of the dopamine system on peripheral immune cells in PD. To further understand the functional consequences of increased DAT+/TH+ PBMCs, we asked if DAT function was altered on PD monocyte-derived cells. Live cell fluorescence microscopy and biochemical analysis confirmed healthy human monocyte-derived macrophages (MDM) express membrane-localized, canonically functional DAT (Km=3.2mM). Surprisingly, relative to healthy age-matched controls, PD patients’ MDMs exhibited dramatically elevated DAT-mediated substrate uptake and increased membrane localization (p<0.0001, n=5 biological replicates). Conclusions: Taken together, these data are consistent with the interpretation that in PD peripheral dopamine homeostasis is dysregulated. Importantly, this presents PBMC DAT/TH as a potential biomarker for PD and suggests the peripheral dopamine system is functionally linked to the CNS dopamine system. Future work will aim to validate this biomarker and investigate the mechanistic connection between peripheral-CNS dopamine systems to elucidate the pathophysiological role of dysregulated monocyte dopamine transmission in PD.
2016
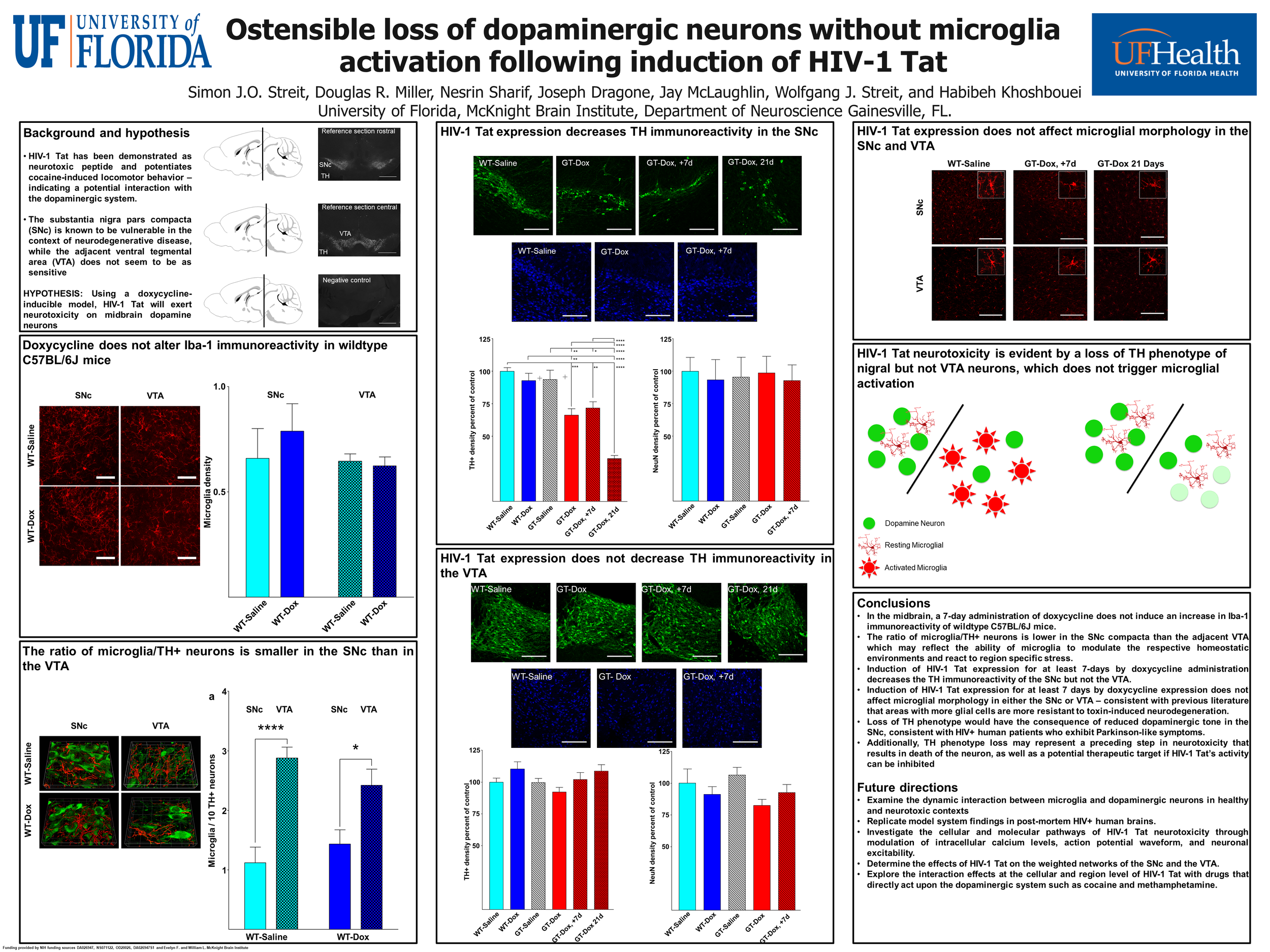
The transactivator of transcription protein, HIV-1 Tat, is linked to neuroAIDS, where degeneration of dopamine neurons occurs. Using a mouse model expressing GFAP-driven Tat protein under doxycycline (Dox) regulation, we investigated microglial-neuronal interactions in the rostral substantia nigra pars compacta (SNc). Immunohistochemistry for microglia and tyrosine hydroxylase (TH) showed that the ratio of microglia to dopamine neurons is smaller in the SNc than in the ventral tegmental area (VTA) and that this difference is maintained following 7-day Dox exposure in wild type animals. Administration of Dox to wild types had no effect on microglial densities. In addressing the sensitivity of neurons to potentially adverse effects of HIV-1 Tat, we found that HIV-1 Tat exposure in vivo selectively decreased TH immunoreactivity in the SNc but not in the VTA, while levels of TH mRNA in the SNc remained unchanged. HIV-1 Tat induction in vivo did not alter the total number of neurons in these brain regions. Application of Tat (5 ng) into dopamine neurons with whole-cell patch pipette decreased spontaneous firing activity. Tat induction also produced a decline in microglial cell numbers, but no microglial activation. Thus, disappearance of dopaminergic phenotype is due to a loss of TH immunoreactivity rather than to neuronal death, which would have triggered microglial activation. We conclude that adverse effects of HIV-1 Tat produce a hypodopamine state by decreasing TH immunoreactivity and firing activity of dopamine neurons. Reduced microglial numbers after Tat exposure in vivo suggest impaired microglial functions and altered bidirectional interactions between dopamine neurons and microglia.
Collaborative Papers
As the resident CNS immune cells, microglia are highly dynamic which functionally influence and are influenced by their microenvironment. In the dopaminergic areas of midbrain, dopamine neurons are the most population of the neighboring cells which directly and indirectly communicate with microglia. To understand cellular mechanisms of this bidirectional interaction, especially during the aging which is a risk factor for the development of Parkinson’s disease, accurate mathematical models are needed to predict the age-related change in the brain of over aged laboratory animals. This research answers three main questions through modeling: (1) how do numbers of microglia (Ibal+), dopamine neuron (TH+), and their ratio (Ibal+/TH+) change during aging in SNc and VTA, (2) how does morphology of microglia change, and (3) is there any difference in the physical contact between microglia and dopamine neuron in SNc and VTA during aging? Brains of five different age groups 1, 6, 9, 18 and 24 months (n=4-5 per each group of age) were fixed, cut, labeled and imaged. Based on general features of data, various models were created for each data set, including polynomials, exponentials and fractions. For each model, treating data as Poisson distribution, maximum likelihood functions were created of which parameters were found by minimizing the Poisson negative log likelihood. Models were evaluated by the Akaike Information Criterion and then selected and weighted based on their Akaike weights. Our result predicted that the number of microglia and TH+ neurons dramatically decreased in over-aged mice (35 months old). Although complexity of microglia declined with age, they make more physical contact sites with dopamine neurons. Projection of model to the age of 30 months suggests ultimate scenario for elderly mice which is usually unattainable through experiment.
Ischemic stroke is a leading cause of mortality and long-term disability. Stroke induces widespread molecular and cellular changes in the brain that are not restricted to the core of the infarct. Delayed secondary damage to the tissue surrounding the stroke core and changes in brain structures distant to the injured region (diaschisis) contribute to neuronal dysfunction. Activated microglia are believed to contribute to neuroinflammation and progressive stroke damage. However, microglial involvement in diaschisis in the ischemic brain remains unclear. We performed morphometric analyses on microglia from ipsilateral and contralateral hemispheres of young and aged mice subjected to middle cerebral artery occlusion. We found a significant increase in the cell body area of contralateral microglia in aged mice, but not in young brains, suggesting a smaller diaschisis in young animals. In contrast, the projection area of contralateral microglia were significantly decreased in both young and aged brains, suggesting a reduced surveillance ability of microglia in structures distant to the stroke core. Sholl analysis revealed a significant decrease in microglia complexity in the contralateral hemisphere of both young and aged mice. Our data support the hypothesis that microglia might be involved in delayed secondary injury to the contralateral side after strokes.
The presence of intercellular pathological inclusions of the protein α-synuclein within dopaminergic neurons is one of the cardinal features of Parkinson’s disease (PD). Healthy dopaminergic neurons have extensive axonal arborization, whereas shrinkage in axonal arborization of dopaminergic neurons is reported in animal models of PD and in PD patients. To examine the contribution of α-synuclein overexpression on the neuronal dysfunction and structure prior to neuronal demise, we performed morphometric analyses on a dopaminergic neuron overexpressing human α-synuclein. We found in comparison to naïve dopaminergic neurons, α-synuclein overexpression significantly decreased neuronal arborization and complexity. Our recent data suggest α-synuclein downregulates D2 receptor activity, and pharmacological activation of D2 receptors restores neuronal activity. Therefore, we examined whether Quinpirole, a D2 receptor agonist, reduces the deleterious effects of α-synuclein overexpression on neuronal complexity, but Sulpiride, a D2 receptor antagonist, accelerates α-synuclein-indued neuropathology. We found D2 receptor agonism but not antagonism restored neuronal complexity suggesting that pharmacological activation of D2 receptors is a potential therapeutic target to alleviate α-synuclein-induced neuropathology prior to neuronal death. Our data support the hypothesis that α-synuclein overexpression contributes to dopaminergic neuronal dysfunction by reducing neuronal complexity, and D2 receptor agonist may provide a potential therapeutic target.